
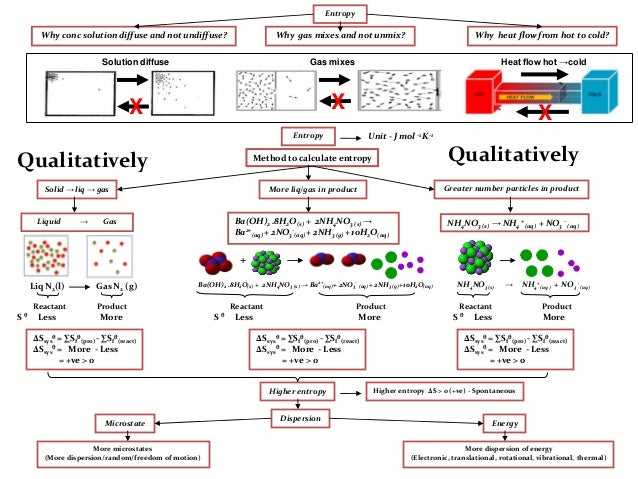
Here we see a glimpse of the beast that is thermodynamics stir under the surface. In short, this is an abstract statement of the second law. The increase in entropy created by decreasing entropy will always be higher.Įntropy never decreases, it strives to attain a maximum.

But, by doing so we use energy and create heat. For example, by freezing water in ice cubes. You are increasing entropy.Ĭan we also decrease entropy? Of course, by storing energy in a more efficient way. If you’ve made it this far, by reading all these sentences you have been generating heat that is transferred to your surroundings.
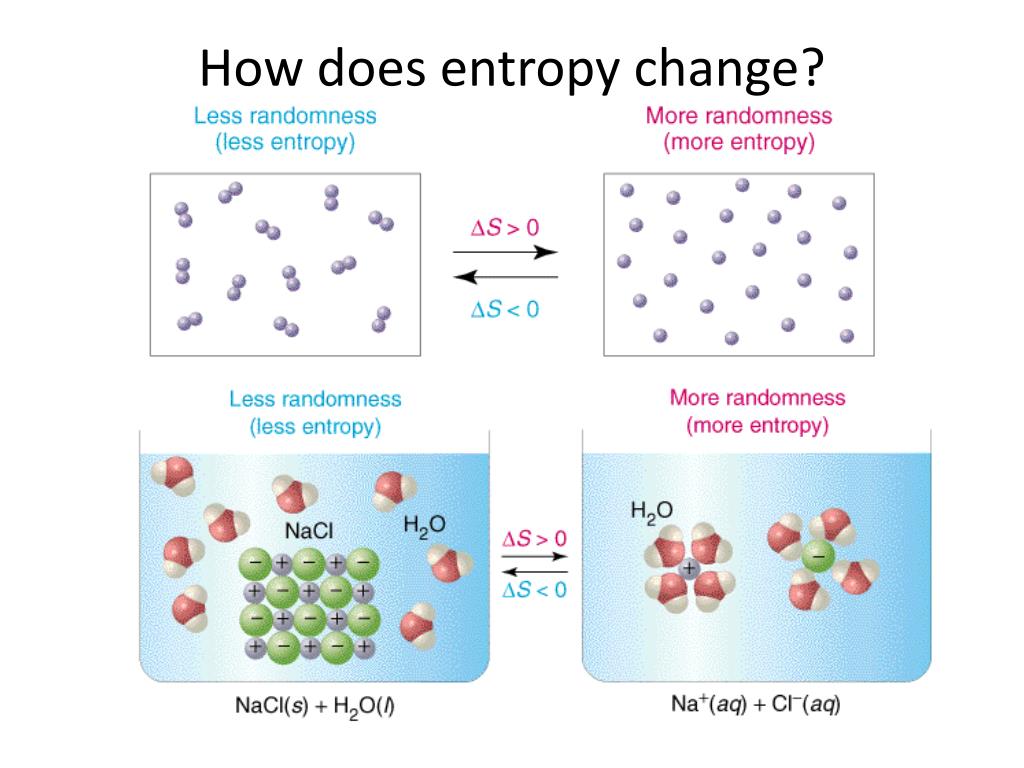
To make things clearer, here is an equation (this’ll be the only equation I promise). In fact, both energy and heat contribute to the change in entropy. Where there is energy, there is almost certainly heat. We all want the most efficient cooling machine, the most economic car, the most efficient way of extracting energy from the resources provided by the earth and sun. That’s a cool analogy, you may think, but what’s in it for me? Why is it important to know energy has different states? Well, this has everything to do with efficiency. Energy stored in a chaotic way (the random-pile library) has high entropy. Energy stored in a carefully ordered way (the efficient library) has lower entropy. Although our two libraries contain the same number of books, they differ in the quality of service they can provide.Įntropy, loosely, is a measure of quality of energy in the sense that the lower the entropy the higher the quality. In another we have a random-stacked pile. In one library, all books are neatly arranged alphabetically on the bookshelves. Just as two libraries contain the same number of books, they may differ in quality. You can call energy the currency of physics.īut one amount of energy is not the same as the other. The first law of thermodynamics tells us that the amount of energy in the universe can never be depleted and nor will it grow.įor example, the energy stored in a log of wood is not lost when you ignite it.
Long story short, entropy says something about the quality of energy. This is the point at which the rules of thermodynamics used to calculate the efficiency of a cooling machine reflect the outcome of the universe. What is entropy? With this simple question we are moving into something far more fundamental, even philosophical. If someone tells you about the pressure and temperature of a cooling machine, it immediately rings a bell.īut if asked about the change in entropy of a system? Well… That’s rather abstract. Temperature, pressure and energy are familiar concepts. Part one: a simple definition Part two: birth of the Second Law Part three: the way the universe will end The most mysterious of all concepts From the cooling in a refrigerator to the formation of a thought. The latter is one of the all-time great laws of science because it tells us why anything happens at all.
What is entropy in chemistry series#
In this blog series we give an easy-to-grasp definition of entropy and th e second law of thermodynamics. The entropy change is unknown (but likely not zero) because there are equal numbers of molecules on both sides of the equation, and all are gases.What is entropy? Part 1: A simple definitionĮver wondered why heat never flows from cold to hot? Why we cannot seem to create a machine with 100% efficiency and why we know our past but not our future? It all has to do with a concept called entropy.


 0 kommentar(er)
0 kommentar(er)
